Kinesis GAIT™
Kinesis GAIT™ enables clinical gait assessment for rehabilitation and research, through the quantitative measurement of movement using wearable sensors.

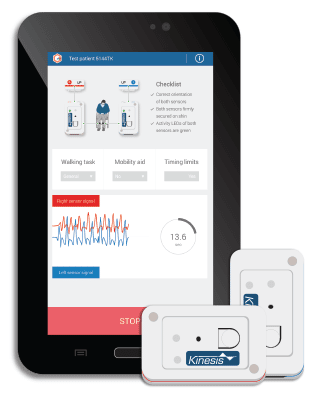
Kinesis GAIT™ enables clinical gait assessment for rehabilitation and research, through the quantitative measurement of movement using wearable sensors. Quantitative gait analysis can be used to identify the presence of gait abnormality, pathological gait or specific gait deviations associated with injury and disease, predict falls in the elderly, and quantify improvements due to rehabilitation. Kinesis GAITTM is a Medical Device: Product is a registered Class I medical device in the EU,under the MDD and carries the CE mark. Kinesis Gait™ is registered with the FDA as a Class I (exempt) medical device in the USA, is registered as a Class I medical device with Health Canada, and is registered with the Therapeutic Goods Administration (TGA) in Australia. Product has been certified for electrical safety to medical device safety standard EN 60601-1.
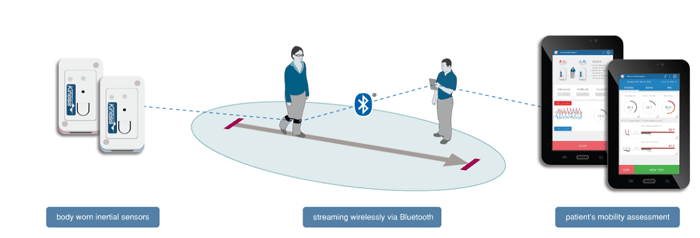
Kinesis GAIT™ Key Features:
NOTE: Content reproduced with permission from Kinesis Health Technologies Ltd.
