CAMBRIDGE, MA – June 25, 2021 – Shimmer Research, a global leader in wearable technology for research applications, today released the initial validation paper for its Verisense® wearable sensing platform for clinical trials. This paper focuses on the Verisense IMU and follows the comprehensive, three-component verification, analytical validation, and clinical validation (V3) framework1 popularized by DiMe.
The Verisense IMU provides raw, tri-axial acceleration data, which is processed via the widely used, open-source GGIR software to calculate clinically meaningful sleep and activity metrics.
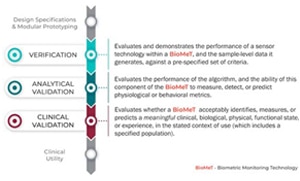
Following the V3 framework is a rigorous process. The initial verification stage involves evaluating and demonstrating the performance of the sensor technology, and the sample-level data it generates, against a pre-specified set of criteria. During the analytical validation stage, the performance of the algorithm is evaluated, together with its ability to measure, detect, or predict physiological or behavioral metrics. Then, in the clinical validation stage, the biometric monitoring technology is evaluated to determine whether it acceptably identifies, measures, or predicts a meaningful clinical, biological, physical, functional state or experience, in the stated context of use (which includes a specified population). An example might be freezing versus tremors in Parkinson’s disease.
“This paper validates the Verisense IMU and provides a practical example of how to employ the V3 framework. It also demonstrates the power and value of open-source solutions,” said Geoffrey Gill, President of Shimmer Americas. “Only the first step of the V3 process is device dependent. The remaining two steps depend on the algorithm, so by using open source, shared algorithms for its Verisense platform, Shimmer allows researchers to leverage any past and future validation studies performed with those algorithms. This creates the opportunity to continually build the industry’s shared body of knowledge. Shimmer is excited to help move this process forward.”
“GGIR, for example, has already been used in more than 150 peer-reviewed papers, covering a wide range of therapeutic areas and patient populations. Furthermore, approximately 50 new papers are published each year employing GGIR – a research rate that no single organization can match,” Mr. Gill added.
“We are delighted that Shimmer has adopted the V3 framework. DiMe is building a library highlighting ‘V3 in practice,’ and we will include this paper along with other leading researchers and innovators using the V3 framework as fundamental to the advancement of high quality digital health measurement products,” said Jennifer Goldsack, Executive Director of DiMe.
The volume of validation data is growing so rapidly that Shimmer plans to update and release new versions of this Verisense IMU validation paper frequently at https://verisense.net/validation-v3-framework. Additional information about the Verisense platform is available at www.verisense.net.
References
1. Goldsack, Jennifer C., Andrea Coravos, Jessie P. Bakker, Brinnae Bent, Ariel V. Dowling, Cheryl Fitzer-Attas, Alan Godfrey et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). npj Digit. Med. 3, 55 (2020). https://doi.org/10.1038/s41746-020-0260-4
2. Migueles, Jairo H., Alex V. Rowlands, Florian Huber, S´everine Sabia, and Vincent T. van Hees. GGIR: a research community-driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. Journal for the Measurement of Physical Behaviour 2, no. 3 (2019): 188-196.
About Shimmer Research
Founded based on Intel technology in 2006, Shimmer Research is a well-established wearable technologies services and sensor manufacturing company based in Dublin, Ireland. In addition to standard products, Shimmer provides customized sensor development services, volume manufacturing, and complete wearable sensor solutions of any complexity. Shimmer’s technology and services have been employed by thousands of researchers at more than 900 leading companies, universities, and research institutes in more than 75 countries. Shimmer’s technology is incorporated in the products and services of more than 20 original equipment manufacturers. Shimmer has an ISO 13485:2016 certified medical devices quality management system. For more information, visit www.shimmersensing.com, https://www.linkedin.com/company/shimmer/ or follow @ShimmerSensing.
Shimmer Contact:
Geoffrey Gill, (617) 945-2628
President, Shimmer Americas
ggill@shimmersensing.com
Media Contact:
Lisa Osborne, (206) 992-5245
Rana Healthcare Solutions
lisa@ranahealth.com