Introduction
Advances in wearable technology have led to the development of many wearable activity trackers for consumers interested in their overall fitness, ongoing health status, and weight management. Wearable devices for the recording of various components of daily activity have been proliferating, but little research has examined how these devices compare with each other and how accurate and effective they are for research and clinical purposes.
Personal wearable trackers cannot be expected to match the utility of research-based devices since they are for different purposes and have different constraints, e.g. ease of use, low cost. Availability of wearable technology offers the possibility of self-monitoring, and may also be relevant for applied field-based clinical trials and intervention programs aimed at encouraging physical activity in the community. Formal assessment of the validity of these devices is important so that consumers, fitness professionals and researchers can make informed choices when selecting one of the monitors.

Methodology
This preliminary validation study was divided into two testing sections, namely standardised activity (SA) and daily living (DL). For the duration of the study the participants wore the Verisense and Actiwatch 2 wrist-worn sensors on their non-dominant wrist. During the SA section participants were asked to sit, walk and jog for periods of 2-6mins. For the DL section, participants wore both sensors continuously for 48h and completed a brief activity diary to correlate to the data collected.
Verisense is a wrist-worn device by Shimmer that measures 3-axis accelerometer and 3-axis gyroscope data to provide in-built activity and sleep metrics.
Integrating Shimmer
The Verisense watches were configured to store data as the integral of activity occurring in 25Hz segments and Actiwatch 2 devices were configured to store data in 15s segments. Time synchronization was performed across the Verisense and Actiwatch devices at the beginning of each participant’s study period. Sleep and activity data was obtained from the Verisense Dashboard and the Actiwatch data was retrieved using Philips Actiware.
During DL testing, all participants simultaneously wore the Verisense and Actiwatch 2 sensors on their non-dominant wrist, continuously over a period of 48 hours in free living conditions (i.e., two nights of data). Participants completed a custom sleep and activity diary concomitant with wearing the accelerometers.

For SA testing, participants simultaneously wore the Verisense and Actiwatch 2 sensors on their non-dominant wrist for the completion of the activities in the gym. Walking and jogging activities where completed on treadmills and across the gym floor. Activities where completed in a randomised order. After each activity, participants stood completely still for 60s with their arms resting freely beside their body.
Initial Results
Data at a sampling rate of 32Hz was generated in 15s epochs from the Actiwatch 2 in the form of time-stamped Activity Counts generated using a proprietary algorithm. Data from Verisense was obtained in the form of raw accelerometer x, y and z axes at a sampling rate of 25Hz. Verisense data was presented with an estimated start time and end time in the file header from which timestamps were calculated.
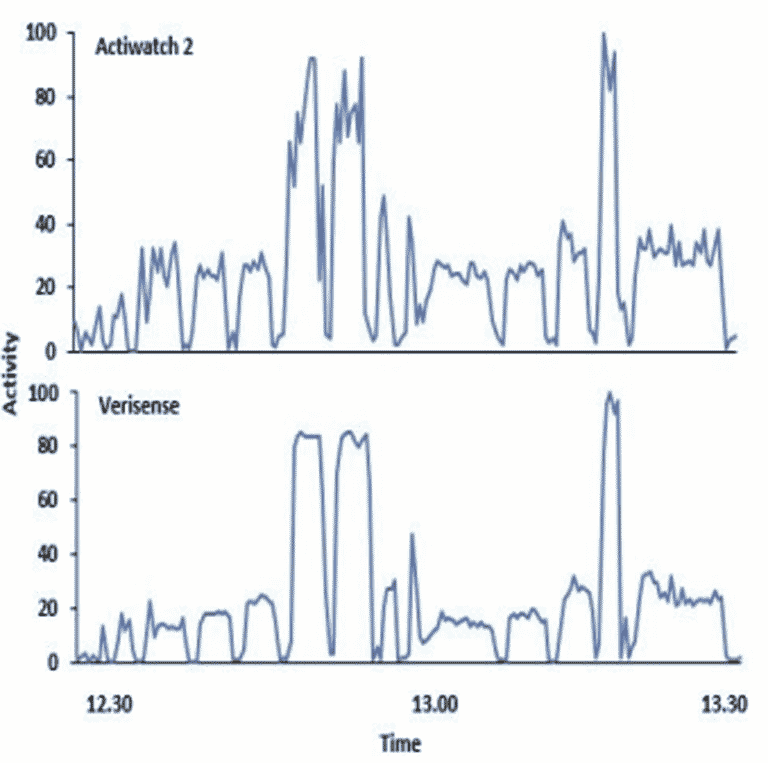
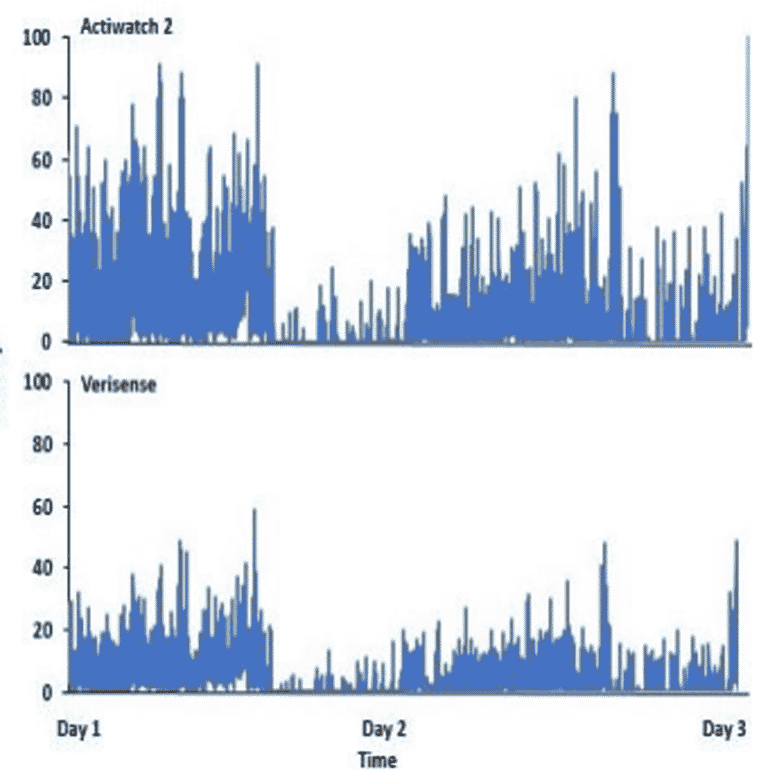
To compare the 2 devices, the Verisense data was transformed to align with the Actiwatch 2. Raw acceleration data from the Verisense was processed using R statistical software and Microsoft Excel. As an initial comparison, data for one participant over 48 hours obtained from both the Verisense and Actiwatch 2 devices show the overall patterns observed between the devices to appear similar.
Clearly aligned spikes in activity show 2-min bursts of vigorous activity periods of movement as supervised in a gym setting with the participant wearing both devices on the nondominant wrist. For example, at approximately 13:15pm, both activity counts and ENMO were very active producing elevated spikes, then both signals gradually declined as an indication of the gym-supervised participant being asked to place their hands by their sides for 1 minute to show a clear delineation of inactivity versus activity. Movement data from gym-based periods were well correlated (r = 0.91; Pearson’s correlation coefficient).
Conclusion and Future Research
This study explores the potential correlation between two activity trackers for activity monitoring. Initial findings illustrate that Verisense provides activity levels comparable to the Philips Actiwatch 2. These findings increase the researchers’ confidence levels in attaining highly correlated results once the timestamps have been harmonized and open up possibilities for the application of Verisense in large clinical studies.
To further the research, the researchers now intend to extend the data gathering component of the study to include more participants and continue to compare the devices using the aforementioned techniques. Sleep metrics will also be evaluated with the devices in order to validate use of Verisense for periods of rest and inactivity. Future findings will add to a growing body of literature on the use of wearable accelerometers for activity monitoring and will assist other researchers in justification of application of such devices in clinical trials.

